Electrodeposition-synthesis of Ni/Cu/Zn nanowires
Electrodeposition is an eletrochemical deposition technique that uses electrical current to reduce the metal cations of a solution and coat a conductive surface. The raw material is a metal salt that is dissolved in an appropriate bath solution. The DC Electrodeposition is used for the synthesis of Cu,Ni and Zn nanowires that grow inside a porous alumina membrane template.
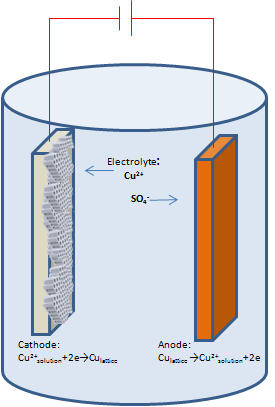
The electrolytic solution consists of copper cations and sulfate anions. With the application of a small electric field, the copper cations migrate to the cathode electrode where they are reduced and deposited as metallic copper as described by the reactionSchematic representation of Cu dc electrodeposition inside a porous alumina membrane.
Nickel nanowires inside porous alumina membranes:
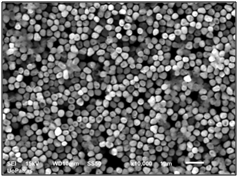
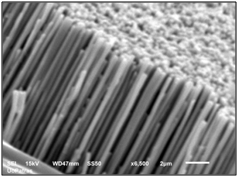
Top view (a) and section view (b) of nickel nanowires perpendicular to silver substrate after porous alumina dissolution. The mean diameter of the nanowires is 240nm.
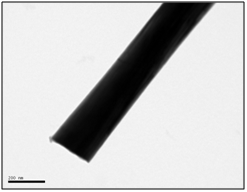

High magnification TEM photo of nickel nanowire and electron diffraction showing a monocrystalline pattern.
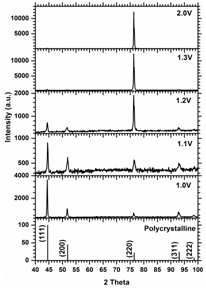
XRD patterns from nickel nanowires fabricated under applied voltage ranging between 1.0V and 2.0V .Crystal structure of nickel nanowires is strongly dependent on the applied voltage. Under low cathodic voltages nickel nanowires with high aspect ratio are polycrystalline with preferred orientation along [111] direction, increasing the applied voltage results to switching of intensities between (111) reflections and (220) reflections. Under 1.3V the nanowires show a monocrystalline structure oriented along [110] direction. The crystal structure of the nanowires remains to be monocrystalline for applied voltages higher than 1.3V.
: