NanoFluidics
For the flow of fluid through a capillary, Poiseuille derived the following relationship between the flow rate (Q ) and the pressure difference (ΔP0) within the capillary:
![]()
where a0 is the pore radius, η is the viscosity of the solvent and L is the pore length. In the presence of an adsorbed polymer layer of thickness δ, the pore is constricted to a diameter a = (a0?δ) and so the above equation becomes:
![]()
where ΔP is the new pressure difference required to maintain the same flow rate. If it is assumed that any changes in the solution viscosity are negligible, then:
![]()
For flow through a membrane it is necessary to assume that the membrane is composed of a number (N) of parallel, uniform cylindrical pores. For the membranes used in these experiments this is a valid assumption and the pore density has been determined to be 1.2*10^13 m^-2, while the mean pore radius is ca. 110 nm.
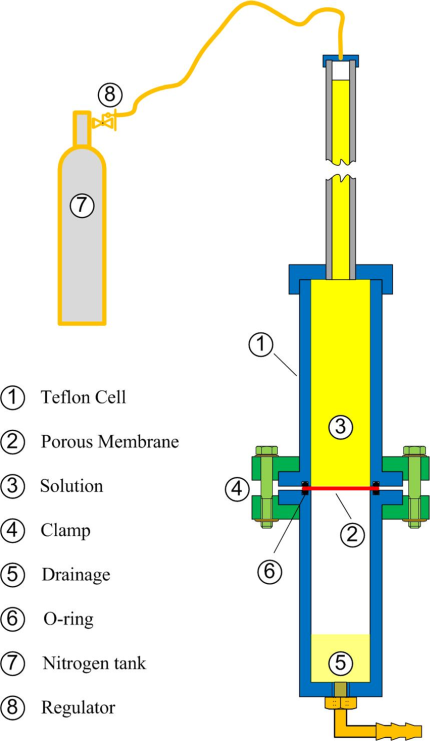
A porous alumina membrane is suitably assembled between a double Teflon cell. Sealing is ensured by a couple of O-rings, while a pressure difference is developed between the upper and lower surface of the membrane by two ways: Hydrostatically and by superimposing an external gas pressure by means of a nitrogen tank and a regulator. The flow rate is evaluated by measuring the time for a certain drop of the surface in a graded glass tube. The pressure is evaluated by adding the hydrostatic pressure that is built up by the solvent above the membrane with the external one that is imposed by the regulator of the nitrogen tank.
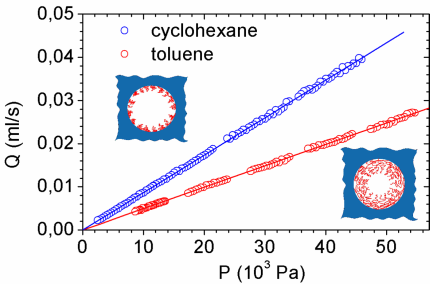
Highly asymmetric PS-PEO block copolymer with long PS chain and short PEO block of 80 kg/mol molecular weight was dissolved in toluene. Adsorption was allowed to take place by first immersing the alumina disk in toluene and then adding solution to the pure solvent so as to obtain the desired bulk concentration, which was 0.5 mg/mL. The membrane was left to adsorb for one week in order to assure saturation as was verified by FTIR spectroscopy in an independent control experiment. The membrane was then removed from the solution, rinsed and assembled in the experimental apparatus. Two sets of measures were taken: one in good solvent (toluene) and one in poor (cyclohexane). The polymer brush that is formed during the adsorption procedure is extended in the good solvent condition (see inset) while is partially collapsed in the poor solvent condition.